Dear customer, please register and verify your account so you can see product price, discounts and make inquiries. Click here to register.
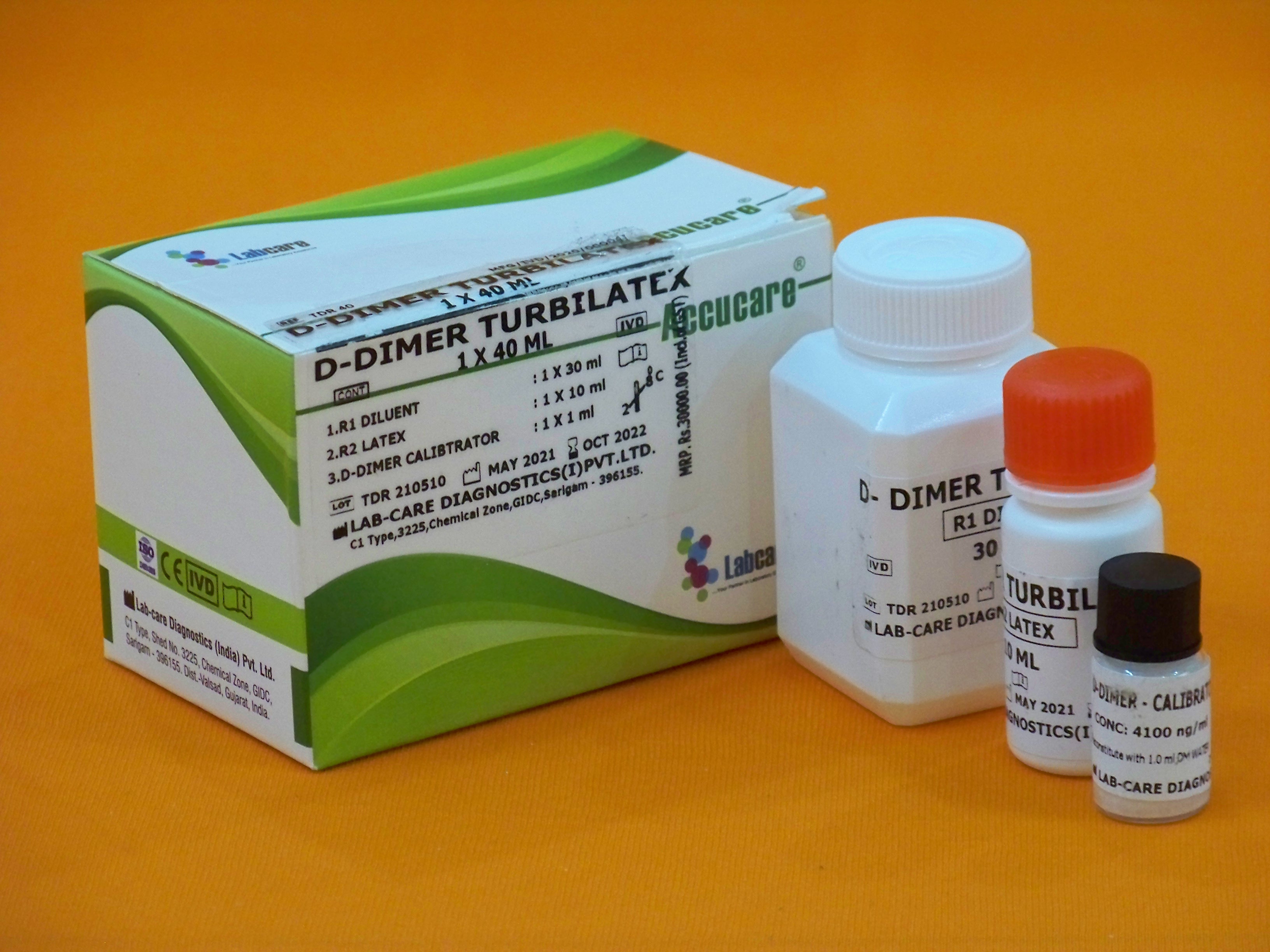
Accucare D-Dimer Turbilatex
Accucare
Dear customer, please register your account with us to know more about price and discount offers on product. Click here to register.
| Brand | Accucare |
R1 Diluent R2 Latex D-Dimer Calibrator | 1x30ml 1x10ml 1x1ml |
- Intended Use: In vitro diagnostic reagents for the quantitative determination of D-Dimer in human Plasma by means of particle-enhanced turbidimetric immunoassay.
- Background: The D-dimer assay is specific for fibrin derivatives. In this assay, the presence of cross linked D-dimer domain is diagnostic for lysis of a fibrin clot, and confirm that thrombin was formed and factor XIII was activated with reactive fibrinolysis. Since fibrinogen derivatives do not contain the cross-linked D-dimer domain, they are not recognized by the D-dimer assay, even when presence in high concentration. In other words, Fibrin derivatives in plasma containing D-Dimer (XDP) are specific markers for fibrinolysis, as opposed to fibrinogenolysis. D-dimers are detected by immunoassays using monoclonal antibodies specific for the cross-linked D-dimer domain in fibrinogen.
- Test Principle: This D-dimer test is based upon reinforced immunoturbidimetry. Monoclonal anti D-dimer antibodies in the reagent react with the D-dimer antigen in the sample, forming antigen/antibody complexes that increase the work solution turbidity.
- Calibrator: Human serum.D-Dimer concentration is stated on the vial label. All raw materials of human origin used in the manufacture of this product showed no reactivity when tested for HBsAg, anti-HIV-1/2 and HCV with commercially available test methods. However, this product should be handled as though capable of transmitting infectious diseases
- Precautions and Warnings: For in vitro diagnostic use only. Do not pipette by mouth. Reagents containing sodium azide must be handled with precaution. Sodium azide can form explosive azides with lead and copper plumbing. Since absence of infectious agents cannot be proven, all specimens and reagents obtained from human blood should always be handled with precaution using established good laboratory practices. Disposal of all waste material should be in accordance with local guidelines. As with other diagnostic tests, results should be interpreted considering all other test results and the clinical situation of the patient.
ddimer biochemistry accucare labcare